Low Level Light Therapy (LLLT) Posted on 12 Oct 05:47 , 0 comments
Several transcription factors are regulated by changes in cellular redox state. Among them redox factor-1 (Ref-1) dependent activator protein-1 (AP-1) (Fos and Jun), nuclear factor κB (NF-κB), p53, activating transcription factor/cAMP-response element-binding protein (ATF/CREB), hypoxia-inducible factor (HIF)-1α, and HIF-like factor. However, it was also shown that low levels of oxidants appear to stimulate proliferation and differentiation of some type of cells (Alaluf et al., 2000; Kirlin et al., 1999; Yang et al., 1996). These effects in turn lead to increased cell proliferation and migration (particularly by fibroblasts), modulation in the levels of cytokines, growth factors and inflammatory mediators, and increased tissue oxygenation (Pastore et al., 1994). The results of these biochemical and cellular changes in animals and patients include such benefits as increased healing in chronic wounds, improvements in sports injuries and carpal tunnel syndrome, pain reduction in arthritis and neuropathies, and amelioration of damage after heart attacks, stroke, nerve injury and retinal toxicity (Hamblin et al., 2006). Figure 10 shows the mechanism and application of LLLT.
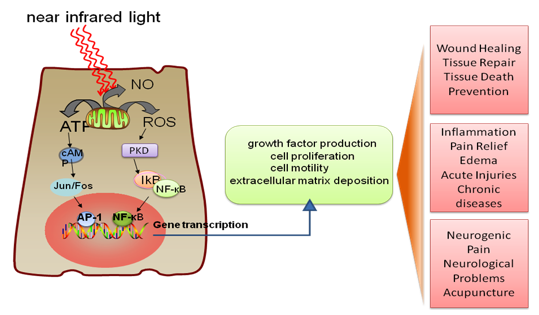
Figure 10. LLLT mechanism and application. Incoming red and NIR photons are absorbed in cell mitochondria, producing reactive oxygen species (ROS) and releasing nitric oxide (NO), which leads to gene transcription via activation of transcription factors (NF-κB and AP1).
9.1. LLLT for pain relief, inflammation and healing. In recent years, there has been growing interest in the use of laser biostimulation as a therapeutic modality for pain management (Strong, 2002). Alterations in neuronal activity have been suggested to play a role in pain relief by laser therapy.
Many published reports document the positive findings for laser biostimulation in pain management. This level of evidence relates to chronic neck pain (Chow et al., 2005), tendonitis (Bjordal et al., 2006), chronic joint disorders (Bjordal et al., 2003), musculoskeletal pain (Gerber et al., 2001), and chronic pain (Aronoff, 1999). Randomised controlled trials provide evidence for the efficacy of laser therapy in chronic low back pain (Frazer et al., 2003).
LLLT has been used clinically since 1981 for the treatment of patients with inflammatory pathologies. LLLT was conducted on different animal models of inflammatory disorders. LLLT have been reported to have a significant effect on carpal tunnel syndrome treatment, mucositis, arthritis, and ulceration.
The literature on LLLT applied to the stimulation of wound healing in a variety of animal models contains both positive and negative results. The reasons for the conflicting reports, sometimes in very similar wound models, are probably diverse. It is probable that applications of LLLT in animal models will be more effective if carried out on models that have some intrinsic disease state. LLLT significantly improves wound healing in both diabetic rats and diabetic mice. LLLT was also effective in X-radiation impaired wound healing in mice. Furthermore, the total collagen content was significantly increased at 2 months, when compared with control wounds. The beneficial effect of LLLT on wound healing can be explained by considering several basic biological mechanisms, including the induction of the expression of cytokines and growth factors known to be responsible for the many phases of wound healing. Figure 11 shows the mechanism of LLLT on wound healing (Lucas et al., 2002).

Figure 11. LLLT for Wound Healing. Cells in the wound respond to light induced reactive oxygen species (ROS) leading to the expression of growth factors, such as transforming growth factor beta (TGF), and platelet derived growth factor (PDGF), which encourage synthesis of more collagen, increased formation of blood vessels, and less inflammation, all of which increase wound healing.
9.2. LLLT in the central nervous system. Low level laser/light therapy (LLLT) for neurological disorders in the central nervous system (CNS) is currently an experimental concept. The broad goals for clinical utilization are the prevention and/or repair of damage, relief of symptoms, slowing of disease progression, and correction of genetic abnormalities. Experimental studies have tested and continue to test these goals by investigating LLLT in animal models of diseases and injuries that affect the brain and spinal cord. Successful clinical trials have been carried out for transcranial laser therapy for stroke. Discoveries concerning the molecular basis of various neurological diseases, combined with advances that have been made in understanding the molecular and cellular mechanisms in LLLT, both in vitro and in vivo, have allowed rational light-based therapeutic approaches for a wide variety of CNS disorders to be investigated.
Neurodegenerative diseases are caused by the deterioration of certain nerve cells (neurons), such as occurs in Alzheimer's disease, Parkinson's disease (Trimmer et al., 2009), and Amyotrophic Lateral Sclerosis (Moges et al., 2009), as well as multiple sclerosis, are all due to neuronal degeneration in the central nervous system (Friedlander, 2003). The chronic, unrelenting, progressive nature of these devastating degenerative diseases has motivated the search for therapies that could slow down or arrest the downward course experienced by most patients, and even more desirable would be a therapy that could actually reverse the neuronal damage. Transcranial light therapy is considered to have the potential to accomplish these goals as depicted in Figure 12. Limitations in knowledge are still apparent, such as the optimal wavelength, light source, doses, pulsed or CW, polarization state, treatment timing and repetition frequency. Collaborative efforts between clinicians and basic researchers will likely increase the usage and understanding of effective laser-based therapies in the CNS (Lampl, 2007).
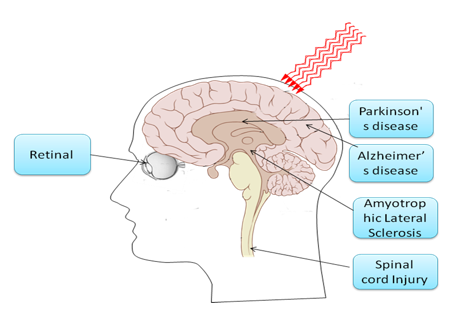
Figure 12. LLLT for central nervous system (CNS) neurological disorders. NIR light can penetrate through the skull into the brain, reducing neuronal cell death, reducing inflammation and increasing the likelihood of neurogenesis. The retinal nerves and the spinal cord are classified as part of the CNS, and light is delivered for similar reasons into the eye or to the neck or back at the site of the spinal cord lesion.
resource from http://www.photobiology.info/Photomed.html
